An international consortium including investigators at MultiClonal Therapeutics, Inc., the Brigham and Women’s Hospital and Harvard Medical School in Boston, the University of Connecticut, the Vanderbilt University, the Jackson Laboratory, the National University of Singapore and the Agency for Science and Technology Research (A*STAR) in Singapore report in today’s on-line version of the British journal Nature the first cloning of “ground state” stem cells of columnar epithelia.
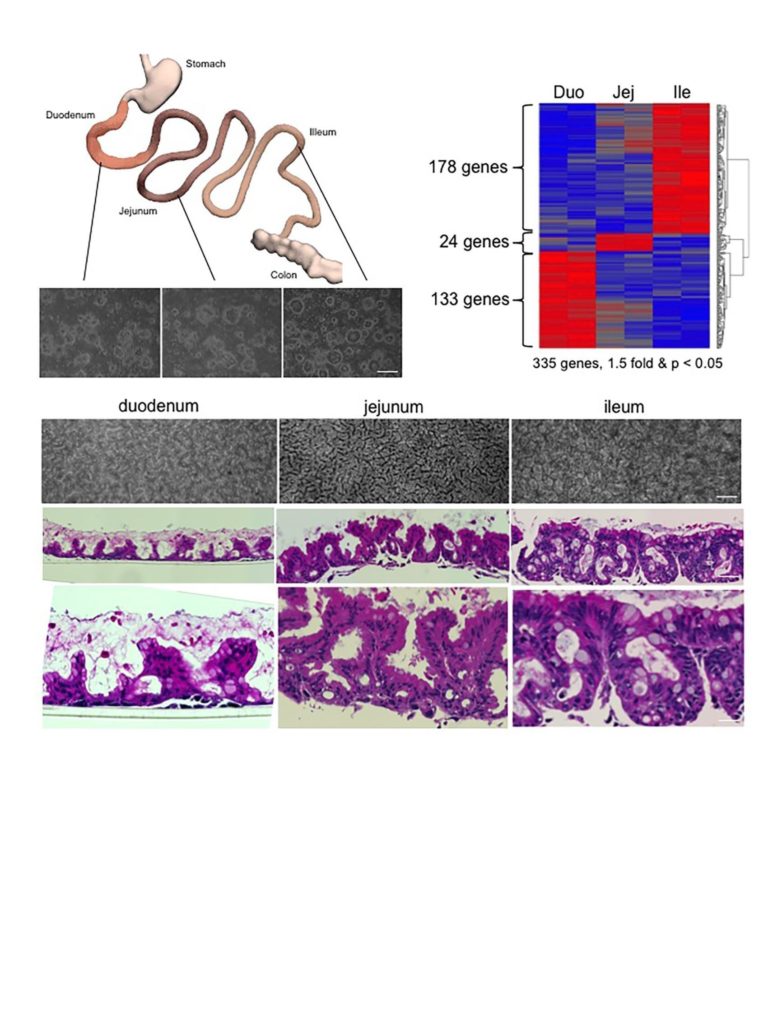
Columnar epithelia includes such medically important tissues of the gastrointestinal tract, the liver, pancreas, and kidney, and a host of other organs central to many human diseases. Up to now, the stem cells of these organs have remained unclonable, thus denying researches and clinicians alike the ability to use them for regenerative medicine and as models of disease processes for new drug discovery. In their absence, biomedical researchers have relied on generating columnar epithelial tissues from so-called “induced pluripotent stem cells (iPSCs)” discovered by Yamanaka and colleagues in Kyoto in 2006. While iPSCs solved many of the immunological and ethical issues surrounding the stem cell field, the past 10 years of iPSC research has uncovered multiple challenges with these cells that the newly cloned adult, ground state stem cells largely circumvent. As corresponding author and visiting professor of Microbiology at NUS and senior group leader at ASTAR and founder of MultiClonal Therapeutics, Inc., Frank McKeon notes, “the properties of these ground state stem cells are truly remarkable. They can be rapidly grown from single cells into billions of cells in several weeks without acquiring significant mutations, remember their precise topological origins in a tissue, and have built-in programs for assembling the appropriate tissues apparently without help from other cells.” The report in Nature focuses on cloning ground state stem cells from the human intestinal tract, a 9-meter tube consisting of functionally distinct segments. The investigators showed that stem cells from each of these segments retain their molecular identity even after 6 months of growth in a laboratory and when asked to differentiate, always form the correct 3-D structures corresponding to their segment of origin. Frank McKeon further remarked “these stem cells are not only genomically stable through many cell divisions but possess a memory for their identity that belies robust epigenetic mechanisms underlying this memory.” It is this memory function of these adult stem cells that provide their greatest advantage over iPSCs, which typically required 10 or more complex interventions to drive them in one tissue as opposed to any other tissue they can make. As notes Wa Xian, communicating author and co-founder of MultiClonal Therapeutics, Inc. , “the exquisite topological specificity of these stem cells is tailor-made for regenerative medicine in the gastrointestinal tract as well as for other organs comprised of columnar epithelia such as the liver and pancreas. Ground state stem cells for these other tissues, especially the liver and pancreas, are a major focus of the Xian-McKeon laboratory working together with colleagues at NUS and other Singapore medical centers”. Lawrence Ho Khek Yu, Professor of Medicine, Vice Dean of Research at NUHS, and author of the present study, notes that “the ground state stem cells offer unprecedented insights into acute and chronic diseases of these organs. This study demonstrates how cloned human colonic stem cells can rapidly answer how the most emergent and problematic enteric pathogen, C. difficile, in fact creates such massive damage to the GI tract.” Prof. Christopher Crum, Vice Chair of Pathology at the Brigham and Women’s Hospital and the Harvard Medical School note that “this technology opens a broad expanse of possibilities for patient-specific disease models and regenerative medicine for some of the most intractable diseases”.
The investigators have already made important inroads using their methods of cloning patient-derived ground state stem cells on inflammatory bowel disease, liver fibrosis and regeneration, as well as various maladies of the pancreas, and foresee a multitude of new therapies for as yet unmet medical needs springing from this work. The vast potential for translating these basic advances in stem cell biology has led to the development by investigators of a new spin-off biotechnology company, MultiClonal Therapeutics, Inc. (MCTx, http://www.multiclonaltx.com) to bring new therapies for chronic diseases such as asthma, COPD, inflammatory bowel disease, and liver fibrosis to the patient.
This research was supported by the Connecticut Innovation, the Joint Council Office of the Agency for Science Technology Research Agency (ASTAR), Singapore, and the National Medical Research Council, Singapore and the National Institutes of Health.
Visit : http://www.multiclonaltx.com